Master Planning
Master planning includes both large scale network analysis, assessing logistics across clients’ site network and supply chain, and individual site planning to future proof operations.

Validation Management
Austin’s Validation Management services can be integrated into live projects or provided independently to improve site’s validation and quality standards.
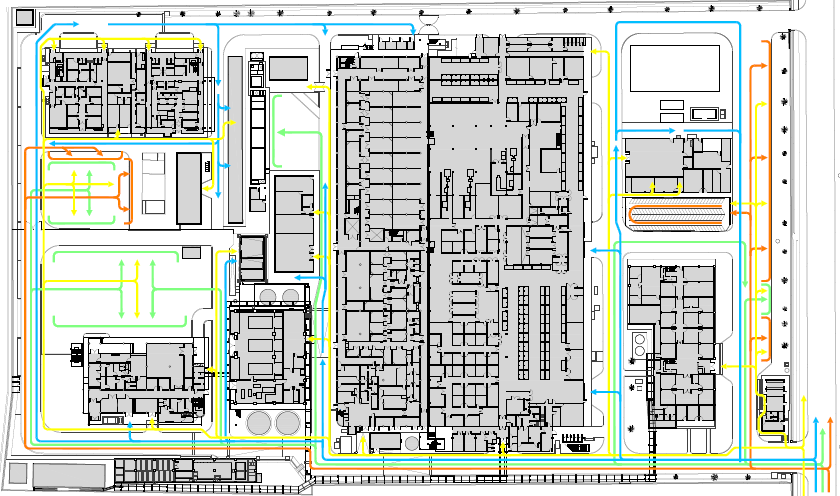
Strategy and Operations
For projects at their earliest stages, when there is a need, but the solution is not yet defined. We provide comprehensive solutions that provide the business case for capital approval.
TO LEARN MORE ABOUT OUR LOCATION CONSULTING PROJECTS, VIEW OUR PROJECT GALLERY